PROBLEM OF ACID RAIN
What is Acid Rain and what causes it?
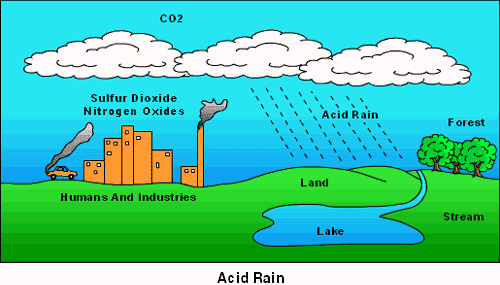
Acid rain is rain that has been made acidic by pollutants in the air. Acid rain can fall on buildings, trees, cars and can make lakes acidic. Humans are the main cause of acid rain since we release many different and dangerous chemicals into the air as you can see on figure 1. Gases like sulfur dioxide and nitrogen oxides can rise very high into the atmosphere and combine with water, oxygen and other chemicals to create acid rain.
http://www.epa.gov/acidrain/education/site_students/whatcauses.html
Figure 1: The causes of Acid Rain
What is Acidity?
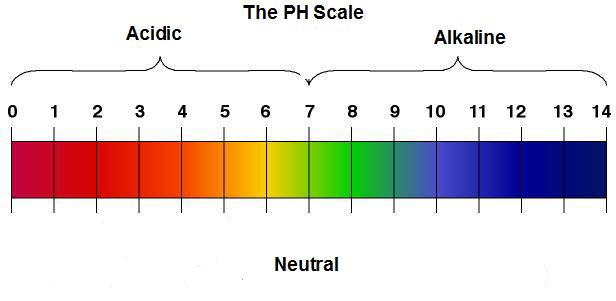
Chemical compounds can be described in two ways, either acidic or alkaline. This is measured by using a pH scale, as shown on figure 2, that runs from 0 (most acidic) to 14(most alkaline) and the middle which is a pH 7 is considered neutral.
http://www.epa.gov/acidrain/education/site_students/whatisacid.html
Figure 2: Typical Acid Rain has a pH level of 4.0.
What effects does acid rain have on the environment?
Forests - Acid rain is very harmful to forests since when its soaked up by the ground it dissolves nutrients such as magnesium and calcium which trees need to be healthy.Figure 3 shows an idea of how trees look as being affected by the acid rain. Also acid rain can cause aluminum to be released into the soil which makes it difficult for trees to take up water.
Figure 3: The damage caused to trees by Acid Rain

Lakes and Streams - Acid rain can make lakes acidic and lower the natural pH level. Also the aluminum that is released into the soil eventually gets into the lakes and streams. This increase in acidity and aluminum levels can kill many aquatic creatures and animals as you can see the pictures on figure 4.
Figure 4: Fish killed by high levels of acidity in the water.

Buildings and Objects - Since acid rain has a high level of acidity it can damage buildings and cars by peeling the paint or destroying the stone and you can see an example of a sculpture on figure 5 as it is destroyed over a period of time.
http://www.epa.gov/acidrain/education/site_students/whyharmful.html
Figure 5: Effects of Acid Rain on Sculptures

What can we do?
Since becoming aware of the acid rain problem, people have created ways to try and prevent acid rain and control it. Regulations have been made on how much sulfur dioxide and nitrogen oxides can be produced by power plants. Also new technology has made it possible to reduce the amount of sulfur dioxide released when burning coal. Car manufactures have also had to make cars that release less nitrogen oxides and are less harmful to the environment. By becoming more aware of this problem, everyone can take action to prevent acid rain by trying to preserve energy and save as much of it as possible.
http://www.epa.gov/acidrain/education/site_students/beingdone.html
Problem: ✔ Pictures: ✔ EBI: The links were hyperlinks
ReplyDeleteCauses: ✔ Remedies: ✔ WWW: Very good pictures with references in text.
Effects: ✔ Hyperlinks: X (There not hyperlinks just links but still there)